Thermal Lensing in Kinetic Measurements
Hyphenation of Thermal Lensing with Kinetic Measurements
The studies of the model indicator system of aniline (phenol) – bromate
(periodate) – vanadium(V) – pyrocatechol/8-hydroxyquinoline under the conditions
of thermal lensing showed that the kinetic-thermal-lens behaviour of the
systems is determined by many new effects, primarily by a change in the colloidal
properties of the medium in the course of the reaction due to the formation
of dispersed particles of the reaction products. 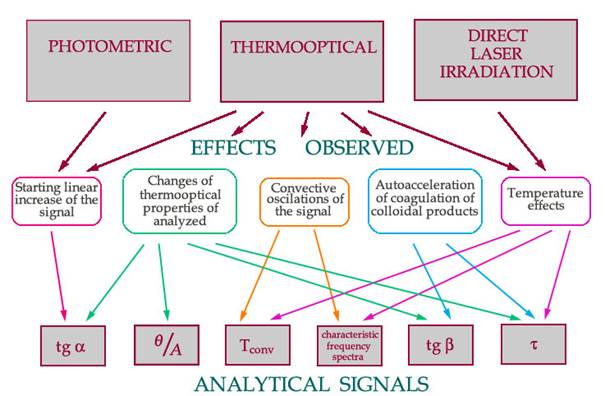
New effects characteristic for thermal detection are summarized in the scheme. New sources of analytical information are chosen (photometric, thermooptical and direct laser irradiation), and the relationships between observed effects and a number of developed analytical signals are presented.
In this area, we try to answer the following questions:
What NEW aspects does thermal lensing bring to kinetic methods of analysis as a DETECTION method?
An increase in the sensitivity and throughput for analytical procedures: A new procedure for determining p-aminophenol in paracetamol dosage formulations was developed (made it possible to decrease the determination time by a factor of 4). The limit of detection is 0.05 ?g/mL , which provides a 20-fold increase in the sensitivity over conventional spectrophotometry.
What NEW can thermal lensing achieve by the COMBINATION in question?
An increase in precision over stationary measurements. The possibility to distinguish between chemical-reaction and convection signals. The combination of kinetics and thermal-lens measurements makes it possible to determine physicochemical characteristics of analytical reactions and clarify reaction mechanisms and fundamental parameters at the trace level of reactants, including oscillatory kinetics.
Is there ANYTHING MORE than just a combination of kinetic methods and thermal lensing as a detector? Will it result in HYPHENATION?
The appearance of new signals combining kinetic and thermal-lens “nature” of the experiments: Studies of model indicator systems of aniline (phenol)–bromate (periodate)–vanadium(V)–pyrocatechol/8-hydroxyquinoline under the conditions of thermal lensing showed that the kinetic-thermal-lens behaviour of the systems is determined by many new effects. The principal possibility of the application of new signals in analytical and physicochemical practice is shown.
Determination of p-aminophenol
in paracetamol samples.
The study of Belousov-Zhabotinsky bromate
oscillators by thermal lensing.
Peculiarities of kinetics-thermal-lens hyphenation.
Determination of p-aminophenol in paracetamol samples
p-Aminophenol is a parent material for the production of paracetamol as one of the most produced pharmaceutical substances worldwide. This compound is also an intermediate product of the decomposition of paracetamol and its analogues in the human body and during the storage of its medicinal preparations. It is a harmful substance for a human organism because it increases body temperature and remains active for a long time. However, existing methods for the determination of p-aminophenol provide only its detection but not quantification. The reaction of p-aminophenol with resorcinol in an alkaline medium was selected as a determination reaction due to the highest reproducibility at the submicrogram level of reactants. Reaction mechanism is the following. The schemes of the reaction of p-aminophenol with resorcinol in alkaline medium.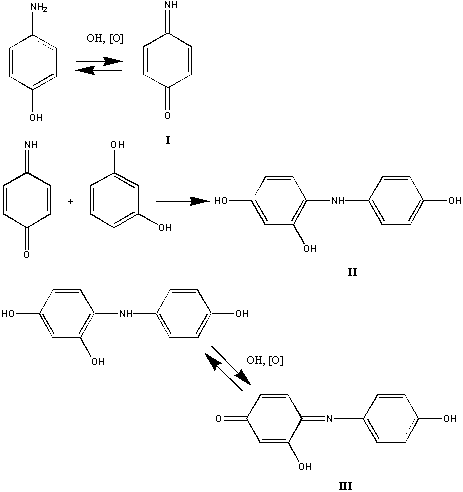
As a result of the electrophilic attack of resorcinol molecule on the indolic form of p-aminophenol in alkaline medium the leuco-dye II is generated. Next, it is oxidized to 2-hydroxyindophenol by air oxygen. The limiting stage is the electrophilic attack, which depends on the concentration of hydroxide ions in the reaction solution. Physicochemical parameters of the reaction (component pseudo-orders, characteristic rate constants, and the activation energy, see the table) were calculated from thermal-lens data, mostly for the first time (see the table), and the reaction mechanism was ascertained compared to existing spectrophotometric data.
Physicochemical parameters of the reaction of p-aminophenol with resorcinol in alkaline medium
| Activation energy, kJ mol-1 | 31.6 ± 0.2 | |
| Characteristic rate constants, L mol-1 s-1 | 293E | 2.10 ± 0.02 |
| 303E | 3.2 ± 0.2 | |
| 313E | 4.8 ± 0.3 | |
| Pseudo-orders, 293K | p-aminophenol | 0.9 ± 0.1 |
| resorcinol | 0.6 ± 0.1 | |
| alkali | 0.4 ± 0.1 | |
| total | 1.9 ± 0.3 | |
From these data the optimal conditions for photometric determination of p-aminophenol by the reaction in question at a submicrogram level were found. The limit of detection by spectrophotometry is 2 µg/mL. Thermal lensing under these conditions provided the limit of detection in neat solutions of 0.1 µg/mL. Thus, a 20-fold increase in the sensitivity was attained. The achieved limit of detection is fivefold lower than the value for the standard procedure for the standard pharmaceutical determination of p-aminophenol with sodium nitroprusside. Also, more favorable conditions of thermal-lens determination made it possible to decrease the analysis time from 40 to 10 min. The studies of the reaction of p-aminophenol with resorcinol in alkaline media in real samples of paracetamol dosage formulations showed that kinetic curves of thermal-lens signal have two linear parts (the picture for the spectrophotometric detection is similar but much less pronounced).
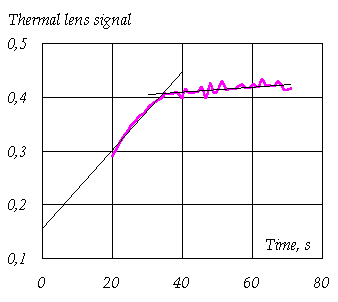
Time dependence of the thermal-lens signal l = 514.5 nm, P = 80 mW, T = 293 K) for the determination of p-aminophenol with resorcinol in alkaline solutions for a real sample of paracetamol.
From this fact and physicochemical characteristics of the processes involved we supposed that two different process coexist in real samples of paracetamol, namely: the interaction of p-aminophenol already existing in the formulation with resorcinol (the first part of the curve) and the decomposition of paracetamol itself to p-aminophenol, which then participates in the reaction (the second part), the rate of the latter process is determined by paracetamol decomposition. Furthermore, an increase in the concentration of OH-ions, which is advantageous for p-aminophenol determination in neat solutions, increases the rate of paracetamol decomposition, which leads to an increase in the error of determination. Thus, new conditions for the determination of p-aminophenol in real paracetamol samples were found by optimising the concentrations of reagents, which provided the lowest rate of paracetamol decomposition (the lowest slope of the second part of the kinetic curve). The procedure was verified by determining p-aminophenol in paracetamol dosage formulations. The limit of detection is 0.05 µg/mL. The content of p-aminophenol in paracetamol formulations made by Farmakon (Russia) is 0.002 wt. %, which is below the minimum allowable concentration stated by the Pharmacopoeia of the Russian Federation A new procedure for determining p-aminophenol in paracetamol dosage formulations was developed (made it possible to decrease the determination time by a factor of 4). The limit of detection is 0.05 mg/mL , which provides a 20-fold increase in the sensitivity over conventional spectrophotometry.
The study of Belousov-Zhabotinsky bromate oscillators by thermal lensing
to the beginningFerroin-catalysed Belousov–Zhabotinsky reaction is widely used in physicochemical and analytical studies. Chemical oscillatory kinetics is gathering the efforts of chemists of various specialities and has recently become a kind of natural laboratory for multidiscipline studies. Kinetic methods based on chemical oscillatory reactions can be applied for very sensitive determination of various substances . However, in both fundamental and applied investigations, it is crucial to pick up a proper kinetic regime and starting parameters and to monitor the reaction conditions. Thus, the selection of the detection method is of importance. We optimised the conditions of the reaction to provide reliable photometric and thermal-lens detection. The studied concentration ranges of components of the bromate oscillator and the found optimum concentrations are summarised in the table.
Concentrations of components (in the final solution) of a bromate oscillator based on iron(II) and its chelate with 1,10-phenanthroline. The concentration of 1,10-phenanthroline is 1 × 10–4 mol dm-3 (spectrophotometric measurements) and 1.25 × 10–6 mol dm-3 (thermal lensing), T = 293K.
| Component | H2SO4 | Fe2+ | Malonic acid | KBrO3 |
| The studied concentration range, mol×dm-3 | 1.0 – 2.0 | 1×10–5 – 2×10–4 | 0.01 – 0.2 | 0.01 – 0.1 |
| Optimum concentration, mol×dm-3 | 1.5 | 1 10–4 | 0.05 | 0.02 |
The most crucial factor is Fe(II):bromate ratio. The concentrations of bromate of 0.01 – 0.02 mol dm–3 and cFe : cBrO3 ratio of 0.005 – 0.01 result in oscillations with a stable period of 40–120 s. Under the optimum conditions, the period is 60 ± 5 s, and the oscillator functions stably for approximately 2 h (see table).
The characteristics of the Belousov–Zhabotinsky reaction or spectrophotometric and thermal-lens studies, T = 293K
| Characteristics | Spectrophotometry | Thermal lensing |
| Period of regular oscillations, s | 60 ± 5 | 100 ± 5 |
| Amplitude of regular oscillations, abs. units | 0.020 ± 0.001 | 0.0050 ± 0.0005 |
| Time interval before the beginning of regular oscillations, min | 2.5 ± 0.5 | 3.0 ± 0.5 |
| Oscillation lifetime, min | 120 ± 10 | 40 ± 2 |
Also, for each of the components (total iron, ferroin, malonic acid, bromate, and sulfuric acid), the dependences of the period of regular oscillations and the oscillation lifetime on starting concentrations of the components (see table).
The orders of dependence of the oscillation period and the oscillation lifetime on initial concentrations of the Belousov–Zhabotinsky reaction for thermal lensing, T = 293K
| Parameter | Fe | [Fe(Phen)3] | BrO3Malonic acid | H2SO4 |
Oscillation
period, x
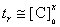 |
–1.5 | –0.12 | –0.5–0.1 | –1.5 |
| Oscillation
lifetime, y |
–0.6 | –0.24 | –1.8–0.2 | –3.5 |
In the range of bromide concentrations 1 × 10–8 – 3.2 × 10–5 mol dm–3, a linear dependence between its concentration and the oscillation period was observed (r = 0.997). Due to the time interval before the beginning of regular oscillations, the measurements of the period for determination of bromide started 3 min after the time of mixing. The calibration equation is described as tr = (800 ± 10) + (90.0 ± 0.5) logcBr (n = 3; P = 0.95), where tr is the period of regular oscillations (in s). The limit of detection (3s) of bromide is 2 × 10–8 mol dm–3 (2 ng ml–1). The repeatability relative standard deviation for the concentration range of bromide of n 10–8 – n × 10–6 mol dm–3 is 2 – 4%.
Peculiarities of kinetic-thermal-lens hyphenation
to the beginningThe studies of the model indicator system of aniline (phenol) – bromate (periodate) – vanadium(V) – pyrocatechol/8-hydroxyquinoline under the conditions of thermal lensing showed that the kinetic-thermal-lens behavior of the systems is determined by many new effects, primarily by a change in the colloidal properties of the medium in the course of the reaction due to the formation of dispersed particles of the reaction products. New effects characteristic for thermal detection are summarized in the scheme. Three sources of analytical information are chosen (photometric, thermooptical and direct laser irradiation), and the relationships between observed effects and a number of developed analytical signals are presented. Figure 2 shows the kinetic behaviour of the aniline-bromate. The catalysed reactions without activators and in the presence of catechol and 8-hydroxyquinoline are different, although under spectrophotometric detection all they are characterized by the same tan a. It results from a change in the colloidal properties of the reaction medium during the formation of reaction products, which depend on the presence and the nature of the activator. It is proposed to use the ratio of thermal lens signal to absorbance (as the function of thermooptical properties), see Figure 3, for monitoring the kinetic behavior. It was found that a thermal-lens kinetic curve for aniline - bromate indicator system after an induction period becomes nearly exponential and rapidly grows to the maximum thermal lens signal. This effect can be accounted for accelerated formation of a colloidal solution of the reaction products in the volume irradiated by a laser beam. New parameters of the curve can be used in developing procedures for simultaneous determination of the participants of the reaction. Thus, a combination of the kinetic-thermal lensing provides more information compared to its spectrophotometric procedure. As a whole, the above examples show that thermal lensing can be used for both determining kinetic parameters and changing performance characteristics of analytical procedures, thus, combining the fundamental and applied research.